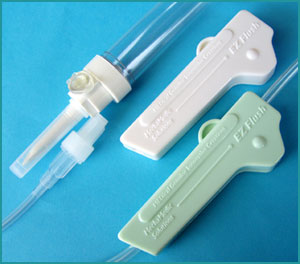
Seeking new medical products and medical product certification Home Page > Articles and Devices > EZ Flush Device
|
||||||||||||||||||||||
| ||||||||||||||||||||||
ISO9001
ISO 13485
CE Mark
FDA Requirements
EP Testing
Class VI
ISO 10993
Home Page Articles and Devices Rubber, Plastics and Silicon Components supplies Medical Thermoplastic Elastomers - Zelas and Tefabloc Blaze Technology: Incure - Medical Cyanoacrylates, sealants and adhesives Suzhou Factory for sale at Wuzhong District Specialty Medical Tubing and Medical Molding HnG Medical Incorporated Standards Certification Contact Us Resources Privacy Site Map